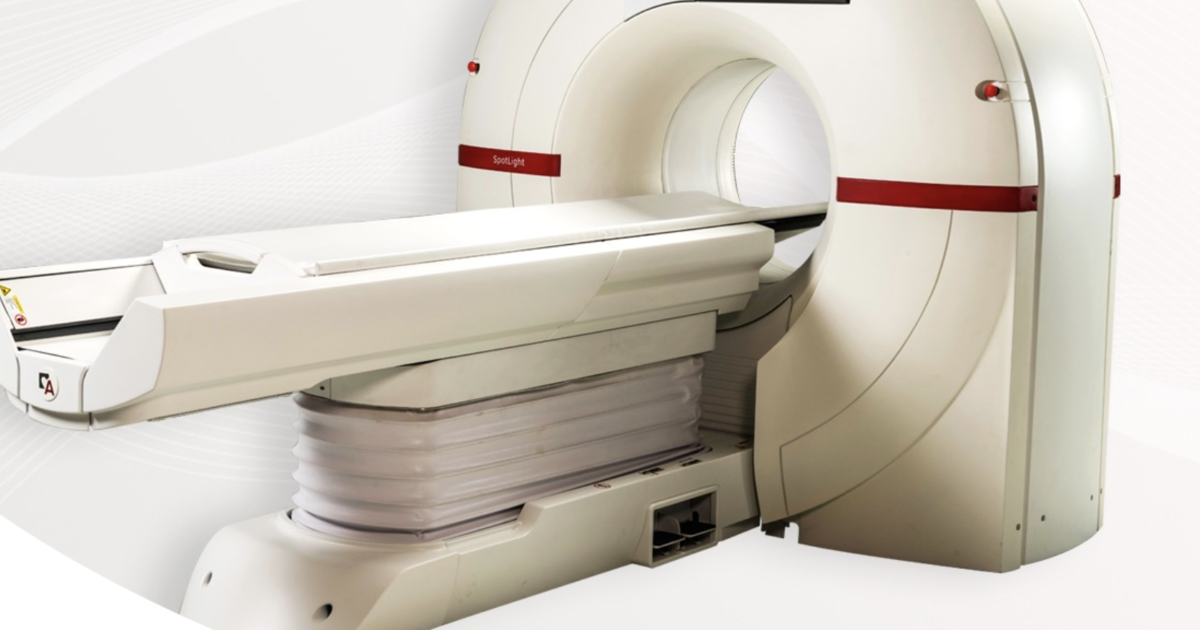
FDA clears cardiac CT scanner for low-dose lung cancer screening
The U.S. Food and Drug Administration has just cleared a cardiac CT scanner for expanded use in lung cancer screenings. Israel-based Arineta—a company that specializes in cardiac CT imaging solutions—announced the 510(k) clearance of its SpotLight Duo …










